PROJECT OVERVIEW:
In this work, we investigate using near-infrared spectroscopy to develop a non-invasive wearable bladder volume sensing system to provide timely alerts to spinal cord injuries (SCI) patients based on their current bladder volume. We showcase the feasibility of such a system using an optical phantom that mimics the bladder and by performing ex vivo measurements on a pig bladder and intestines.
MOTIVATION:
Many patients who suffer from SCI also suffer from neurogenic bladder dysfunction and lack the sensation and control of their bladder. While the prevention of renal failure is of paramount importance, the primary day-to-day concern of most patients is continence. In order to alleviate the buildup of bladder pressure from urine production and promote good renal health, it is recommended to perform clean intermittent catheterization (CIC) every 2 to 4 hours throughout the day. Without timely CIC, the bladder will fill with urine to capacity and eventually leak.However, since urine production in humans is not constant, sometimes the bladder will fill with urine to capacity before the recommended CIC time causing the patient to leak, adding unnecessary embarrassment. Incontinence can have major impacts on patients’ social and mental health, and thus, on their quality of life. As such, the primary concern of many SCI patients is incontinence. Sadly, there are no practical solutions available on the market that addresses this concern.
STATUS AND PRELIMINARY RESULTS
Device:
Our device consists of a non-invasive light probe, an embedded system and a custom software for data acquisition, display and analysis. Our device contains optical components needed to estimate the amount of urine present inside the bladder at a given instant. This is done by transmitting light in the near-infrared spectrum through the abdomen to then measuring the signal strength through an optical receiver. The receiver is connected to a custom electronic circuitry for signal amplification and filtering after which the signal is transferred back to the UI for data visualization.
Testing on Phantom:
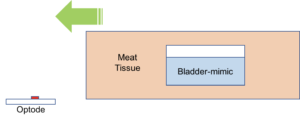
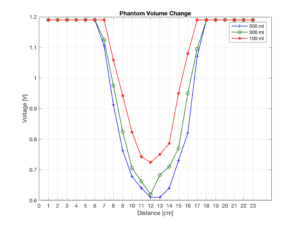
To test our device we have developed an optical phantom to mimic the gross anatomy and optical properties of the bladder and its surrounding environment. As shown, the bladder is represented with an 8cm container filled with water, and its surrounding tissue is represented using a mixture of bovine muscle and fat with a thickness of 2cm between the optical probe and the bladder representation. For the measurements, we slide the optical phantom across the optode (fixed) with 1 cm increments. The same experiment was performed at different volumes of water inside the container to mimic bladder contraction and expansion. As the amount of water inside the bladder is increased more infrared signal is absorbed by the bladder and hence the signal strength at the receiver decreases.
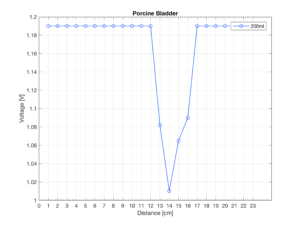
Testing on Porcine Bladders:
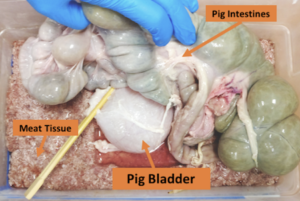
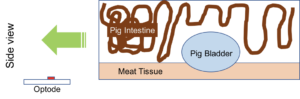
As a step towards investigating a more realistic bladder environment, we worked with a campus-affiliated abattoir to obtain a pig bladder and intestines immediately post-mortem to collect ex vivo measurements using our system. As illustrated, the bladder was surrounded by the intestines in order to create a more anatomically accurate depiction of the bladder environment. Using a system of syringes, tubes, and clamps we filled the pig bladder with 200ml of water and the measurements were taken using our device.
The following figure shows our device being successfully able to detect the location of Porcine bladder in the above mentioned setup:
PLANNED NEXT-STEPS:
At this point we have an IRB approved investigating evaluation on humans.
Some of our planned next steps for our project include performing a human study on patients coming to UC Davis Medical Center for their urodynamic study. We are simultaneously testing the device on healthy subjects at the UC Davis Campus.
On the device end, we are looking to merge the microcontroller and the add-on shields into a single embedded board which is battery operated and has the ability to data transfer data wirelessly through Bluetooth.
ACKNOWLEDGEMENTS:
This research is supported by the CITRIS seed funding award 2017-154 (http://citris-uc.org/). We would also like to acknowledge support from Banatao Institute at the University of California and would like to thank the UC Davis Meat Lab for their help with obtaining tissue samples. UC Davis College of Engineering and the UC Davis Medical Center.
References:
- E. A. Gormley, “Urologic complications of the neurogenic bladder,”Urologic Clinics of North America, vol. 37, no. 4, pp. 601 – 7, 2010.
- L. S. Nahm, Y. Chen, M. J. DeVivo, and L. K. Lloyd, “Bladder cancer mortality after spinal cord injury over 4 decades,” The Journal of Urology, vol. 193, no. 6, pp. 1923 – 1928, 2015.
- J. Coosemans and R. Puers, “An autonomous bladder pressure monitoring system,” Sensors and Actuators A: Physical, vol. 123–124, pp. 155 – 161, 2005, eurosensors fXVIIIg 2004. The 18th European Conference on Solid-State Transducers.
- S. Majerus, A. S. Basu, I. Makovey, P. Wang, H. Zhui, C. Zorman, W. Ko, and M. S. Damaser, “Wireless bladder pressure monitor for closed-loop bladder neuromodulation,” in 2016 IEEE SENSORS, Oct 2016, pp. 1–3.
- E. A. Kiely, G. G. Hartnell, R. N. Gibson, and G. Williams, “Measurement of bladder volume by real-time ultrasound.” Br J Urol, vol. 60, no. 1, pp. 33–35, Jul 1987.
- M. Dicuio, G. Pomara, F. Menchini Fabris, V. Ales, C. Dahlstrand, and G. Morelli, “Measurements of urinary bladder volume: Comparison of five ultrasound calculation methods in volunteers,” vol. 77, Apr 2005.
- A. J. Macnab, R. E. Gagnon, and L. Stothers, “Clinical nirs of the urinary bladder – a demonstration case report,” Spectroscopy, vol. 19, no. 4, pp. 207–212, 2005.
- B. Molavi, B. Shadgan, A. J. Macnab, and G. A. Dumont, “Noninvasive optical monitoring of bladder filling to capacity using a wireless near-infrared spectroscopy device,” IEEE Transactions on Biomedical Circuits and Systems, vol. 8, no. 3, pp. 325–333, June 2014.
- F. Jobsis, “Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters,” Science, vol. 198, no. 4323, pp. 1264–1267, 1977.
- L. Kou, D. Labrie, and P. Chylek, “Refractive indices of water and ice in the 0.65- to 2.5-um spectral range,” Appl. Opt., vol. 32, no. 19, pp. 3531–3540, Jul 1993.
- S. A. Prahl, “Tabulated molar extinction coefficient for hemoglobin in water,” Accessed: May 2017. [Online]. Available: http://omlc.org/spectra/hemoglobin/summary.html
- R. L. van Veen, H. Sterenborg, A. Pifferi, A. Torricelli, and R. Cubeddu, “Determination of vis- nir absorption coefficients of mammalian fat, with time- and spatially resolved diffuse reflectance and transmission spectroscopy,” in Biomedical Topical Meeting. Optical Society of America, 2004.
- R. Wilson, K. P Nadeau, F. B Jaworski, B. J Tromberg, and A. Durkin, “Review of short-wave infrared spectroscopy and imaging methods for biological tissue characterization,” vol. 20, p. 30901, Mar 2015.
- C. Rose, A. Parker, B. Jefferson, and E. Cartmell, “The characterization of feces and urine: A review of the literature to inform advanced treatment technology,” Critical Reviews in Environmental Science and Technology, vol. 45, no. 17, pp. 1827–1879, Sep 2015.